The tumor suppressor p53 is inactivated in virtually all cancers, including leukemias, by mutations or deletion of the TP53 gene, or overexpression of negative regulators (e.g., MDM2, MDM4, and XPO1). MDM2 and MDM4 are frequently overexpressed in acute myeloid leukemia (AML), with the highest MDM4 expression as compared to other malignancies. XPO1 transports ~300 proteins, including p53, from the nucleus to the cytoplasm and MDM2 is also a cargo protein transported by XPO1.We previously reported high synergism by MDM2 and XPO1 inhibition in AML (Kojima et al., Blood 2013), with the underlying mechanism yet to be fully investigated.
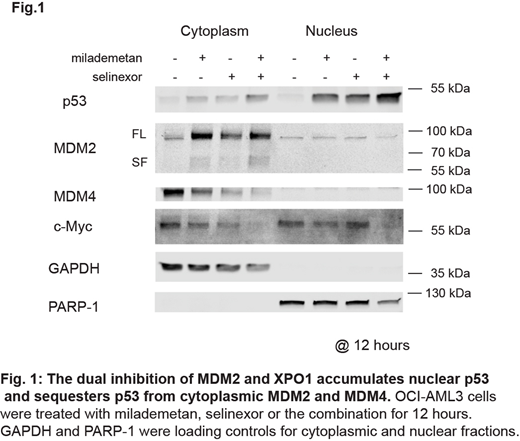
Wp53 was highly accumulated in the nucleus by combined treatment in OCI-AML3 and primary AML cells with MDM2 inhibitor milademetan (DS-3032b) and XPO1 inhibitor selinexor (KPT-330), compared to treatment with individual drugs. Upon MDM2 and XPO1 inhibition, MDM2 was exclusively localized in the cytoplasm, not in the nucleus. Intriguingly, MDM4 also localized exclusively in the cytoplasm, and the dual inhibition markedly reduced the level of cytoplasmic MDM4 (Fig.1). Data suggest that the dual inhibition of MDM2 and XPO1 maximizes the transcriptional activity of p53 by sequestering MDM2 and MDM4 in the cytoplasm, with massive p53 induction in the nucleus. Indeed, the combination treatment dramatically induced p53 targets CDKN1A and MDM2 (i.e.,55-fold and 25-fold, respectively).
WPathway analysis from RNA seq of OCI-AML3 cells treated with milademetan, selinexor, and the combination revealed the TP53 pathway was the top upregulated pathway compared with control, or single agent treatments. E2F targets, G2M checkpoint genes and MYC targets were the principal downregulated pathways by the combination treatment. Cell cycle analysis measuring EdU/DNA, Ki-67, p53, p21, and active caspase-3 revealed elimination of S-phase and a population with the highest Ki-67 levels at G2/M phase, with increased percentages of cells in G0 and G2/M phases. The combination treatment markedly reduced Ki-67 levels, suggesting the disruption of DNA synthesis and cell cycle arrest. Furthermore, p53 and p21 levels were increased in both G0 and G2/M cells, along with increased active caspase-3 levels, suggesting apoptosis induction in both highly proliferating and quiescent AML cells.
WNext to validate c-Myc inhibition by the combination treatment, we used OCI-AML3 cells transduced with shRNA control (ShC) and shRNA for p53 (Shp53), and MOLM-13 cells with wild-type p53 (WT) and with TP53 p.R248W/R213* mutation (MT), obtained through long-term exposure to MDM2 inhibitor. c-Myc protein levels were significantly reduced in OCI-AML3 ShC cells and MOLM-13 WT cells, but not in OCI-AML3 Shp53 cells or MOLM-13 MT cells. Combined treatment synergistically reduced MYC mRNA and c-Myc protein levels both in the cytoplasm and the nucleus. Consistently, the combination treatment reduced c-Myc levels in primary AML cells with wild-type TP53 as opposed to those with TP53 mutations. Overexpression of c-Myc in OCI-AML3 cells conferred increased susceptibility to the combination treatment. Finally, c-Myc protein levels at baseline had negative correlation with the respective ED50 concentrations that induced apoptosis in 50% of AML blasts.
In conclusion: we identified strikingly increased transcriptional activity of p53 which was retained in the nucleus and sequestration of MDM2 in the cytoplasm as novel mechanisms of combined MDM2/XPO1 inhibition. In addition, high c-Myc baseline levels were associated with response to the combinatorial treatment, which also markedly reduced nuclear and cytoplasmic c-Myc levels due to MYC repression by p53 activation. These findings support the translation of combined MDM2 and XPO1 inhibition into clinical trials.
Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lesegretain:Daiichi-Sankyo Inc.: Current Employment. Shacham:Karyopharm: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: (8999996, 9079865, 9714226, PCT/US12/048319, and I574957) on hydrazide containing nuclear transport modulators and uses, and pending patents PCT/US12/048319, 499/2012, PI20102724, and 2012000928) . Andreeff:Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding; Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal